Abstract
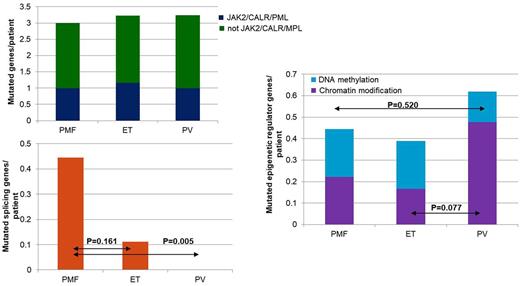
Myeloproliferative neoplasms (MPNs) comprise of a group of clonal stem cell disorders characterized by proliferation of red blood cells (RBC; polycythemia vera or PV), platelets (essential thrombocytosis or ET) or white blood cells (WBC; primary myelofibrosis or PMF). Biggest challenge with MPNs is the leukemic transformation that occurs in 1-2%, 4-5%, and 20-30% of patients over a 10-year period in ET, PV, and PMF, respectively. These secondary leukemia patients are associated with dismal prognosis, with median survival of 6 months. Mutations in JAK2 have been observed in the majority of MPN patients, and though JAK2 mutation seems to be the phenotypic driver of MPNs, recent evidence suggest clonality and mutational events antecedent to JAK2 are responsible for leukemic transformation. To better understand the impact of somatic mutations that contribute to transformation of MPN to leukemia, and the clonal architecture of these mutations, we conducted this study. The collection of bone marrow samples, saliva samples and clinical data of 48 clinically JAK2 positive MPN patients (i.e. JAK2 V617F PCR positive) was carried out at Seoul National University Hospital, and this study was approved by the institutional review board. Written informed consents were obtained from all patients in accordance with the Declaration of Helsinki. The bone marrow samples obtained at chronic MPN state were subjected to target sequencing. Target genes providing diagnostic or prognostic information in myeloid malignancies were selected, and the final gene panel consisted of 2228 amplicons covering 47 genes. Amplified targets were sequenced on the Illumina TruSeq Amplicon. The mean coverage depth was 1056X. For 3 patients with sequential paired samples at MPN diagnosis and at secondary transformation, whole genome sequencing (WGS) was performed using Illumina HiSeq2000. Fisher's exact test was used to compare the mutation frequency of single genes in different MPN subgroups, while Levene's test was used to for comparison of mutation frequency of gene groups. Two-sided t-test was used to evaluate the statistical significance. There were 18 ET patients (37.5%), 21 PV patients (43.8%) and 9 PMF patients (18.8%), and the median age at MPN diagnosis was 63 years for the total cohort. Not surprisingly, PMF was associated with most secondary transformations (P=0.030). About 89.6% of the patients carried somatic mutations other than JAK2. The most frequently observed mutations included TP53, PTCH1, ASXL1, TET2, CALR, EZH2, KMT2A, MPL and SETBP1. Interestingly, majority of the patients carrying ASXL1 were PV. PMF patients more frequently carried mutated splicing genes per patient (p=0.005), while PV patients seem to carry more mutated epigenetic regulator genes per patient, especially the chromatin modifiers (P=0.077). In addition, somatic mutations in TP53 and NF1 are associated with increased risk of secondary transformation and these mutations seem to be present already in the chronic MPN state at a low allelic burden. The WGS results showed the number of mutations at chronic MPN state versus at secondary transformation did not significantly differ, suggesting novel mutation rate is low in MPN and the secondary transformation is indeed driven by expansion of already existent small clones. Efforts to effectively incorporate next generation sequencing into MPN diagnosis and follow-up will be helpful for recognition of patients at high risk of secondary transformation, and can ultimately lead to better clinical outcomes.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal